Epizootic Hemorrhagic Disease Review
Epizootic hemorrhagic disease is a viral disease affecting white tailed deer, mule deer, and reindeer in North America and can cause significant mortality. It is transmitted by biting midges (Culicoides spp.), also called no-see-ums or punkies. It is not spread directly from deer to deer and humans cannot be infected by contact with deer or bites from midges.
EHD outbreaks are most common in the late summer and early fall when the midges are abundant. The cycle stops within two weeks from the onset of frost when the midges and virus are killed.
Results from Experimental EHD Vaccine Use
Medgene has been generating data for the EHD vaccine since 2016. In 2020, we received USDA approval to provide our experimental EHD V2 and V6 vaccine. Vaccine users collected data regarding safety and clinical impression of the vaccine. Prior to 2016, data was shared with the industry at conferences and through magazine articles. In 2020, Medgene worked with a Missouri veterinarian to evaluate the EHD V2 and V6 vaccine in young fawns.
The vaccine was administered to ten (10) 2-3 week old fawns. Blood serum was collected pre (Day 0) and post (Day 35 and Day 63) vaccination to monitor the immune response. A plaque reduction neutralization assay was performed to determine the neutralizing antibody titer of each fawn to both EHD V2 and EHD V6 at varying time points. A titer greater than 10 indicates that antibodies are present that neutralize the virus. The presence of antibodies provides evidence that the fawn generated the intended immune response to the vaccine. (Table 1)
| Table 1. EHD V2 Vaccine Immune Response Generated in Young Fawns | |||||||
|---|---|---|---|---|---|---|---|
| EHDV2 Neutralizing Antibody Titer | EHDV6 Neutralizing Antibody Titer | ||||||
| Tag # | 13Jun2020 | 4Jul2020 | 01Aug2020 | 13Jun2020 | 4Jul2020 | 01Aug2020 | |
| W8 | 20 | 40 | 80 | <10 | 40 | 40 | |
| W9 | <10 | 40 | 40 | <10 | 40 | 40 | |
| W10 | 20 | >320 | 160 | <10 | 40 | 80 | |
| W11 | <10 | >320 | 80 | <10 | 80 | 80 | |
| W12 | <10 | >320 | >320 | <10 | 40 | 40 | |
| W13 | <10 | 160 | 80 | <10 | 40 | 80 | |
| W14 | 80 | >320 | >320 | <10 | 80 | 160 | |
| W15 | 40 | >320 | >320 | <10 | 40 | 80 | |
| W16 | 20 | 80 | 10 | <10 | 80 | 20 | |
| W17 | <10 | 80 | 20 | <10 | 80 | 10 | |
| W18 (Control) | <10 | <10 | <10 | <10 | <10 | <10 | |
| Vaccination dates: 13 June 2020 and 4 July 2020 | |||||||
To further our understanding of the serological response to booster vaccination, the Missouri fawns continued to be evaluated throughout 2021. To review, in 2020, the farm vaccinated ten (10) 2-3 week old fawns. In 2021, they revaccinated those same animals (10 months old when 2021 booster 1 was given; 13 months old when 2021 booster 2 was given) and Medgene Labs monitored the antibody response. Table 2 summarizes EHDV2 antibody titers after booster vaccination.
The data indicates that booster vaccination is critical for increasing the level of neutralizing antibodies to the target disease.
Medgene continues to work with this Missouri farm and these animals to determine the optimal booster schedule. Our current recommendation after the administration of the first two initial doses is to booster every six months, or twice each spring. The goal is to ensure a high antibody level when EHD season arrives.
| Table 2. EHD V2 & V6 Vaccine Immune Response Generated in Young Fawns | |||||||
|---|---|---|---|---|---|---|---|
| EHDV2 Neutralizing Antibody Titer | |||||||
| Tag # | 17 Apr 2021 | 15 May 2021 | 14 Jun 2021 | 10 Jul 2021 | 7 Aug 2021 | 4 Sep 2021 | 2 Oct 2021 |
| W8 | 160 | <10 | 160 | 160 | >320 | >320 | 80 |
| W9 | <10 | 40 | 160 | >320 | >320 | >320 | >320 |
| W10 | 80 | 160 | >320 | >320 | >320 | >320 | >320 |
| W11 | 20 | 80 | >320 | >320 | >320 | >320 | >320 |
| W12 | 160 | >320 | >320 | >320 | >320 | >320 | >320 |
| W13 | 20 | 80 | >320 | >320 | >320 | >320 | >320 |
| W14 | >320 | >320 | >320 | >320 | >320 | >320 | >320 |
| W15 | 160 | >320 | >320 | >320 | >320 | >320 | >320 |
| W17 | 10 | 20 | 80 | 160 | >320 | 160 | 80 |
| W18 (Control) | 10 | 10 | 40 | 80 | 80 | 10 | 80 |
| Vaccination dates: 13 Jun 2020, 4 Jul 2020, 2 Apr 2021 and 10 Jul 2021 | |||||||
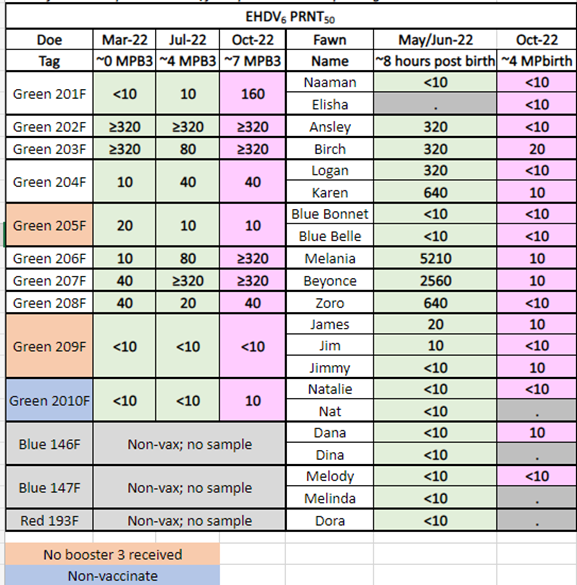
In 2022, Dr. Knibb allowed Medgene to continue to use these does, and their fawns, for further vaccine evaluation. The does were given a single booster vaccine on March 19, 2022. Fawns were allowed to consume colostrum and then blood collected for serological evaluation. The data generated from fawns of vaccinated does proved that maternal antibody, derived from vaccination, passed to fawns. However, by 4 months of age, the fawns lost most of the maternal antibody, indicating vaccination would be needed to protect fawns from EHD in the fall. Tables 3 and4 present the results.
Table 3. EHDV-2 PRNT50Titers in Fawns from Vaccinated Does
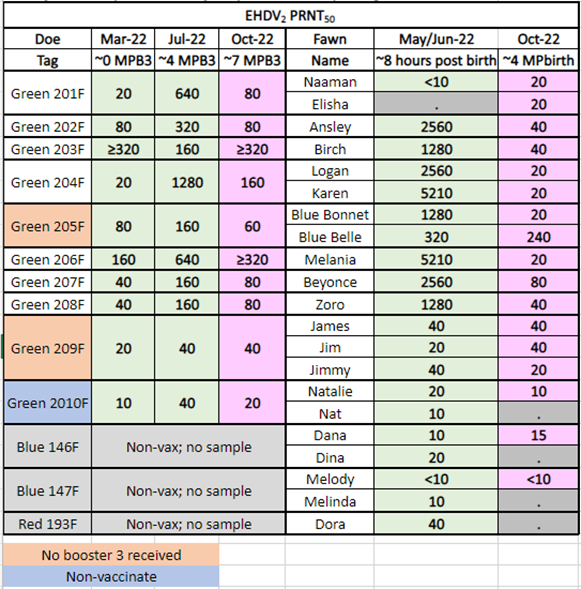
<10 indicates no detectable antibody titer. ≥320 indicates the titer was as high as or higher than the test endpoint.
Table 4. EHDV-6 PRNT50Titers in Fawns from Vaccinated Does